ECJ on health claims for botanicals
Following the reference by the German Federal Court of Justice to I ZR 109/22 and the opinion of the Advocate General, which we reported on, the ECJ has now ruled on 30 April 2025 in C-386/23 in preliminary proceedings on the question of the applicability of on-hold claims to botanicals. The decision is far less negative than the reports on it (and their reach, even on orf.at) would suggest.
According to Article 13(1) of the HCVO, there are three categories of health claims (apart from risk reduction claims and claims for children), namely:
- Claims about the role of a nutrient or other substance in growth, development and the functions of the body (lit a)
- Claims about psychological and behavioural functions (lit b)
- Claims about the slimming or weight-control properties of a food (lit c)
These details are treated differently in the transitional provisions; the claims underlying the original dispute concerned details relating to the psychological and behavioural functions.
- "mood-enhancing saffron extract"
- "The saffron extract Safr'Inside in [the product in question] was tested on 50 participants over a period of 30 days in an open study. With a dose of 30 mg of Safr'Inside per day, 77% of participants experienced improved emotional balance after just two weeks of use and felt more optimistic and happier. 66% also felt more relaxed and energetic. After 30 days, 11% of participants reported improved sleep quality."
- “Melon juice extract with superoxide dismutase activity has been shown in studies to reduce feelings of stress and exhaustion after four weeks. In addition, irritability and exhaustion were reduced by 63%, leading to a significant improvement in quality of life.”
The reffering court had doubts as to whether the transitional provisions were still applicable in general. It considered the transitional provision in Article 28(6) HCVO to be relevant in this case.
Article 28(6) regulates transitional provisions in two groups of cases:
- Claims that have been assessed and approved in a member state.
They are evaluated by the Commission and either approved or rejected. If they are rejected, they may still be used for six months after the decision. If no assessment is made (on hold), they may therefore continue to be used, provided that their effectiveness is proven and, in general, Article 5 of the HCVO is complied with.
The Standing Committee on the Food Chain and Animal Health Section on General Food Law stated in a meeting in February 2010 (Summary Record of Meeting of February 22, 2010, no longer available) that inclusion in the list pursuant to Article 13(2) should be sufficient as an assessment and authorization within the meaning of point (a):
„Finally, as the Commission raised the issue of the different transition provisions applicable respectively to Article 13(1) a), and 13(1) b) and c) claims, Member States confirmed that submission of Article 13(1) b) and c) claims in accordance with Article 13(2) was considered sufficient in terms of observing the transition regime referred to in Article 28(6) (a). (Quoted from: Holle/Hüttebräuker/Conte-Salinas, 1st edition 2018, Regulation (EC) No. 1924/2006, Art. 28, beck-online).
The ECJ did not address this aspect and only considered Article 28(6)(b) to be relevant. This is understandable, as the claims in question are not on the on-hold list.
2. If these claims were not assessed and approved at national level, an “application” had to be submitted under the HCVO before January 19, 2008..
The application must be explained in this context: Health claims pursuant to Article 13(1)(b) and (c) were part of the collective procedure for drawing up the Article 13 list. No formal application was submitted by the user of health claims in this respect. An individual authorization, which is granted upon application, was or is only relevant in this respect for “new” data or for an application for data protection. It is therefore questionable whether, for purely formal reasons, an application pursuant to Article 13(5) had to be submitted for such claims in order to be able to invoke the transitional period (for a detailed discussion of this issue, see Bruggmann, LMuR 2007, 97). If the inclusion of health claims in the national lists pursuant to Article 13(2) is not already classified as a national evaluation and authorization (Article 28(6)(a)), inclusion in the list will at least have to be sufficient as a requirement for the “application” pursuant to letter b (see also Meyer, in: Meyer/Streinz, Explanatory Notes HCVO, para. 132). As discussed in para. 31, this problem has been alleviated with the publication of the partial list of Art. 13 claims: Art. 28(6)(b) is now only relevant for health claims pursuant to Art. 13(1)(b) and (c) that are “on hold,” i.e., that relate in particular to botanicals (→ Art. 10 margin note 8, 18 et seq.). (Holle/Hüttebräuker/Conte-Salinas, 1st edition 2018, Regulation (EC) No. 1924/2006, Art. 28, margin note 35, beck-online)
The Hamburg Higher Regional Court found—without this being challenged in the appeal—that Novel Nutriology had not submitted an application for the melon juice extract contained in the product in question and that its application for the ingredient saffron extract dated January 13, 2009.
The so-called “on-hold list” provided by EFSA on its website for download at download is relevant for the assessment of botanicals. There is no entry for melon juice extract or saffron and the above information. The information at issue was therefore not part of the list used in practice to determine on-hold claims. Incidentally, the predecessor to the list published by EFSA was a list that is no longer available containing the IDs of pending on-hold claims:
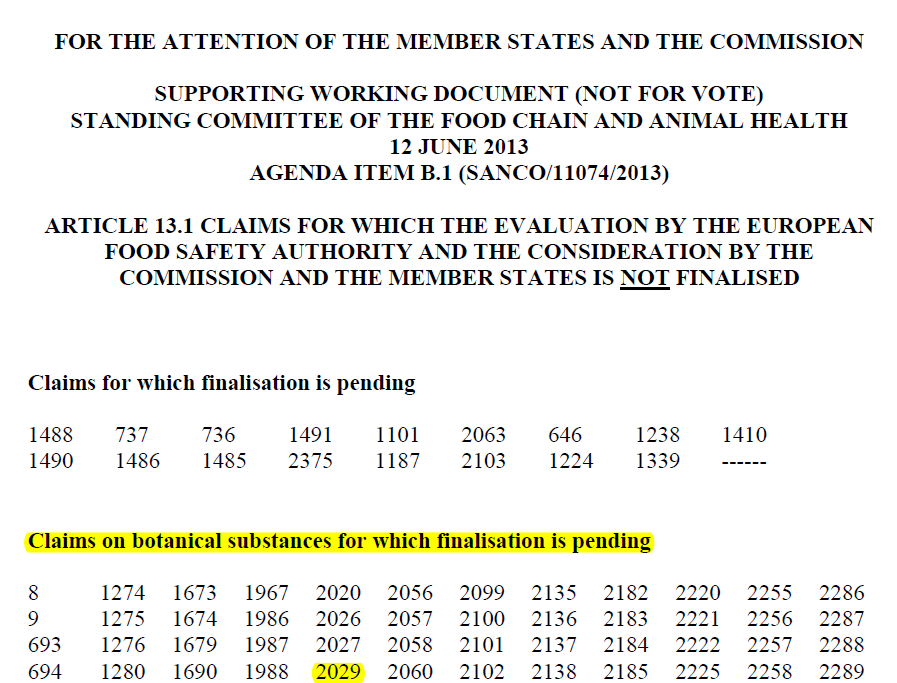
This supports the view that, for the purposes of assessment and approval under Article 28(6)(a), and in any event for an application under Article 28(6)(b), inclusion in the lists of the Member States is sufficient.
Accordingly, it remains essential to only use those on-hold claims (under your own corporate responsibility and with proof of effectiveness) that are included in the on-hold list. In my opinion, this is also the prevailing practice in consulting.
Equally positive is the conclusion in paragraph 66 that Article 10(3) HCVO also applies to on-hold claims that may be used under the transitional provisions. The “addition” of an on-hold claim to justify a statement under Article 10(3) is therefore permissible.
Botanical claims therefore remain an interesting option for promoting food supplements in particular—provided that their effectiveness can be proven.


